How RNAi Therapeutics Work
What is RNA interference (RNAi)?
RNA interference (RNAi) is a natural biological process that regulates gene expression (how your body makes proteins) by "interfering" with messenger RNA (mRNA) which carry DNA's instructions for making new proteins.
How RNA interference (RNAi) Therapeutics Work
Our medicines use RNA interference to "silence" gene expression for specific proteins that have been discovered to cause or contribute to diseases. Our RNAi therapeutics mimic the RNA interference process by delivering specially designed small interfering RNA (siRNA) that, join with a protein complex already in the body called RISC (RNA-induced silencing complex), to target and degrade specific mRNA before they can deliver their instructions, by cleaving them like a pair of molecular scissors.
Different from other types of medicines?
Our RNAi therapeutics act before unwanted proteins are made compared to many other classes of medicines which target proteins after they've been made. Additionally, RNAi therapeutics can be administered infrequently — every three or six months for example.
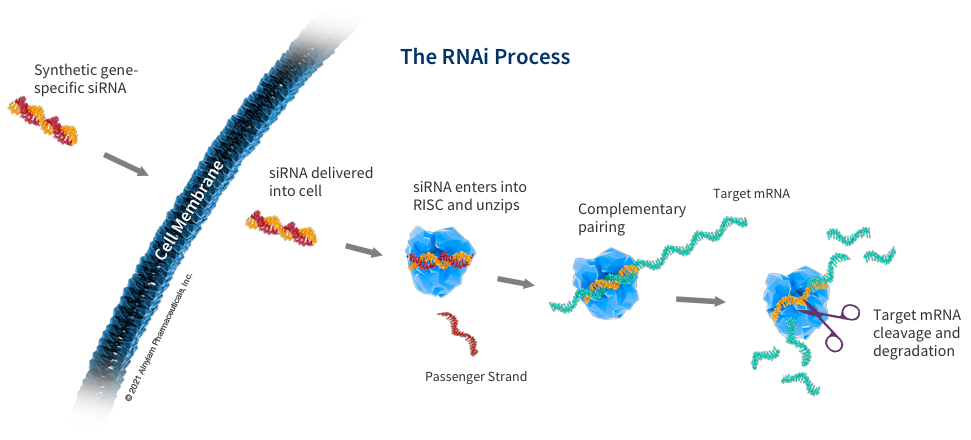
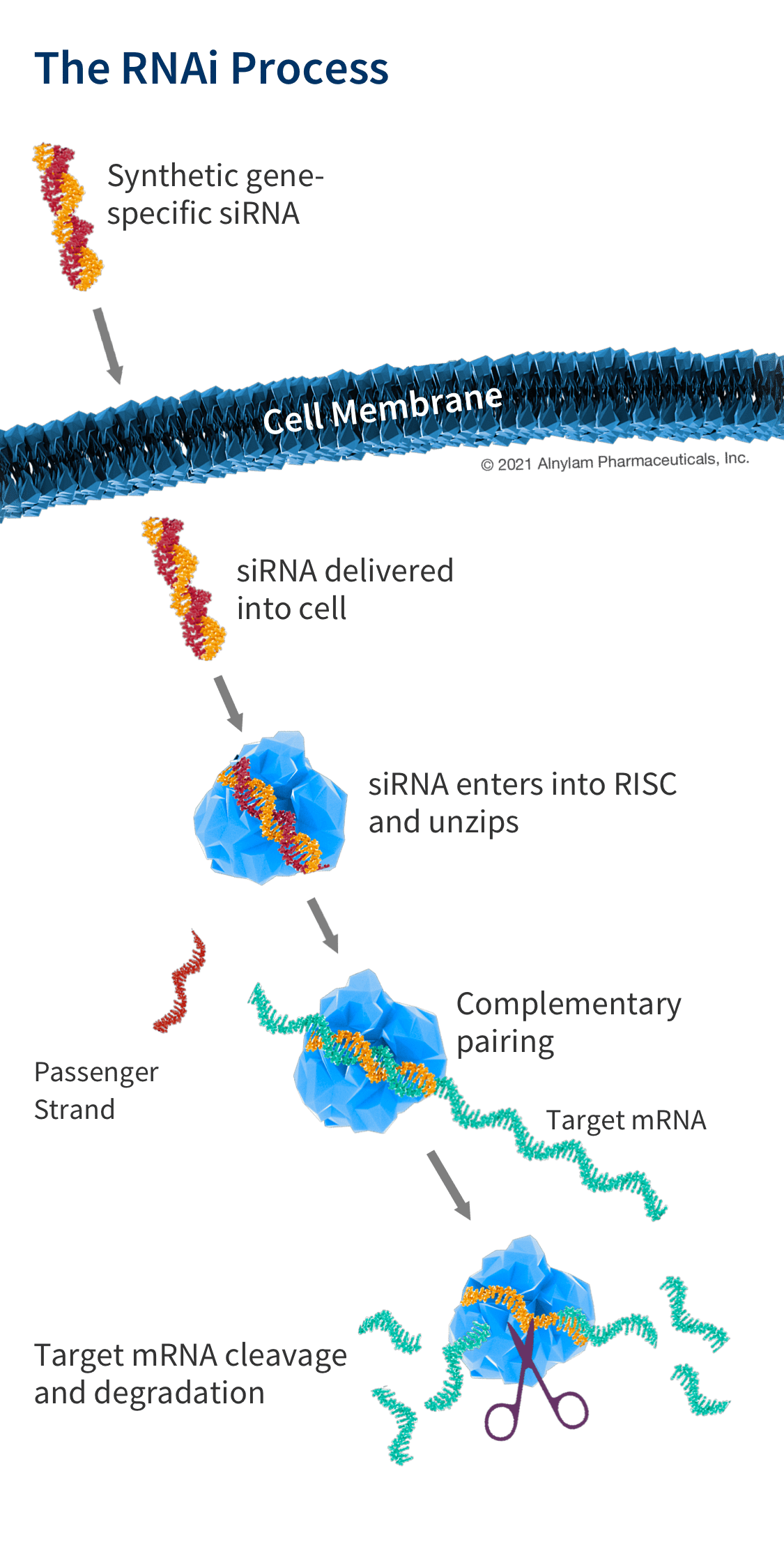
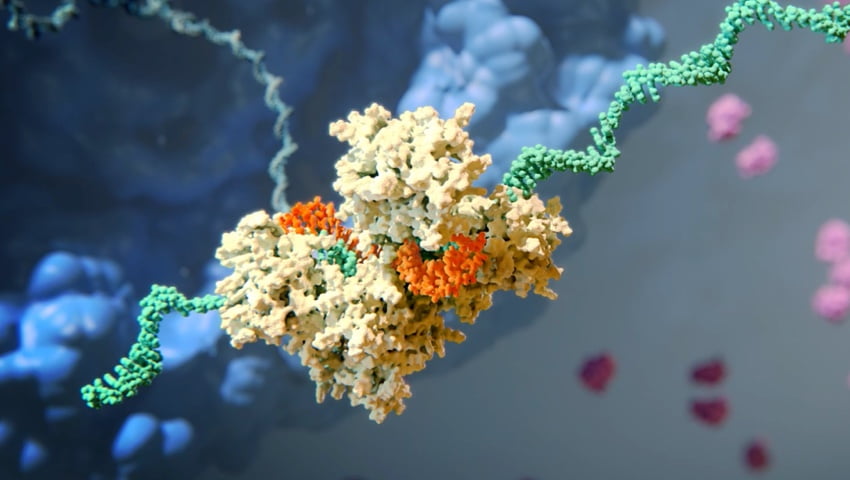
How siRNA Work
When the siRNA duplex is delivered into the cell, it is recognized by a protein complex known as the RNA-induced silencing complex (RISC), which already resides in the cell as a primary component of the natural RNAi pathway. Our siRNA duplex is recognized by and loaded into RISC, which removes one of the two strands (the “passenger” strand). This functional RISC now has only the complementary (or “guide”) strand that stays bound to the RISC, helping it find and pair with its matching (or “complementary”) mRNA before it is converted into a protein by a ribosome. Once the match is found, like a pair of molecular scissors, the siRNA together with RISC cleaves the “unwanted” target mRNA, causing it to be degraded. This process is catalytic, meaning that a single siRNA-loaded RISC can degrade many copies of the target mRNA. As a result, the production of the specific “unwanted” protein that corresponds to that mRNA is reduced or “silenced.”
Additionally, we believe that siRNA can be developed to address infectious diseases by directly targeting viral RNA or their host factors for destruction such that the virus is unable make copies of itself or to get inside cells in the first place.
Key Features of our RNA interference therapeutics:
- Ability to target potentially any gene in the genome, including targets that are “undruggable” by small molecules and antibodies
- Highly potent and durable effect (dosing as infrequently as biannual or annual)
- Administration through multiple routes—intravenous (IV), subcutaneous, and intrathecal delivery
- Demonstrated clinical benefit with a lower dose and dose frequency, and an encouraging overall safety profile compared to other approaches to gene silencing
- Modular, reproducible, and consistent performance across organs and diseases
*out-licensed
Our Pipeline
Learn about how we are leading the translation of RNAi (RNA interference) into a whole new class of innovative medicines.



You will be redirected to an external site that may include social media, event registration, or other third-party content related to Alnylam.
Please note that these external sites have their own terms of service and privacy policies, which may differ from those of Alnylam.
Proceed to Site