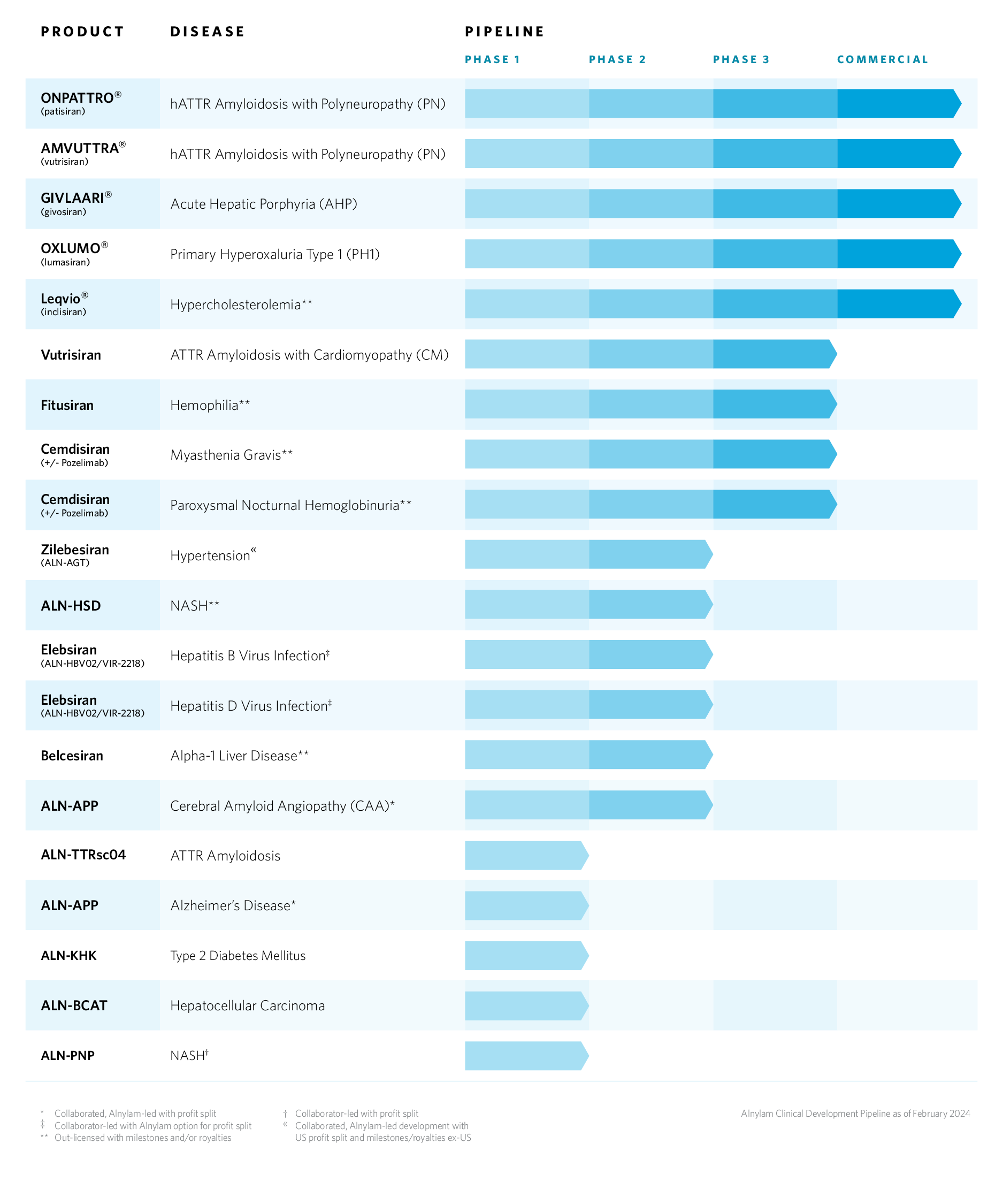
Alnylam Clinical Development Pipeline
Learn more about the latest in Alnylam news from Capella—the destination for updates on our progress in translating the science of RNAi to innovative potential medicines.
SIGN UP FOR EMAIL UPDATES
SIGN UP FOR EMAIL UPDATES
Receive news and updates on the work at Alnylam that affects you most.
Custom Body Class
our-pipline-capella
