hATTR Amyloidosis At-A-Glance
hATTR Amyloidosis Inside The Body
hATTR Amyloidosis Perspectives

Early diagnosis is very important.
– Hannah, living with hATTR amyloidosis
Additional Information

The Bridge
The Bridge is a program designed to help raise awareness of hATTR amyloidosis and promote education on the condition for patients and their families.

Alnylam Act®
Alnylam Act® provides no-charge, independent genetic testing and counseling to individuals in the US and Canada who may have hATTR amyloidosis.
The Bridge, Alnylam Act, and their associated logos are trademarks of Alnylam Pharmaceuticals, Inc. © 2023 Alnylam Pharmaceuticals, Inc. All rights reserved.
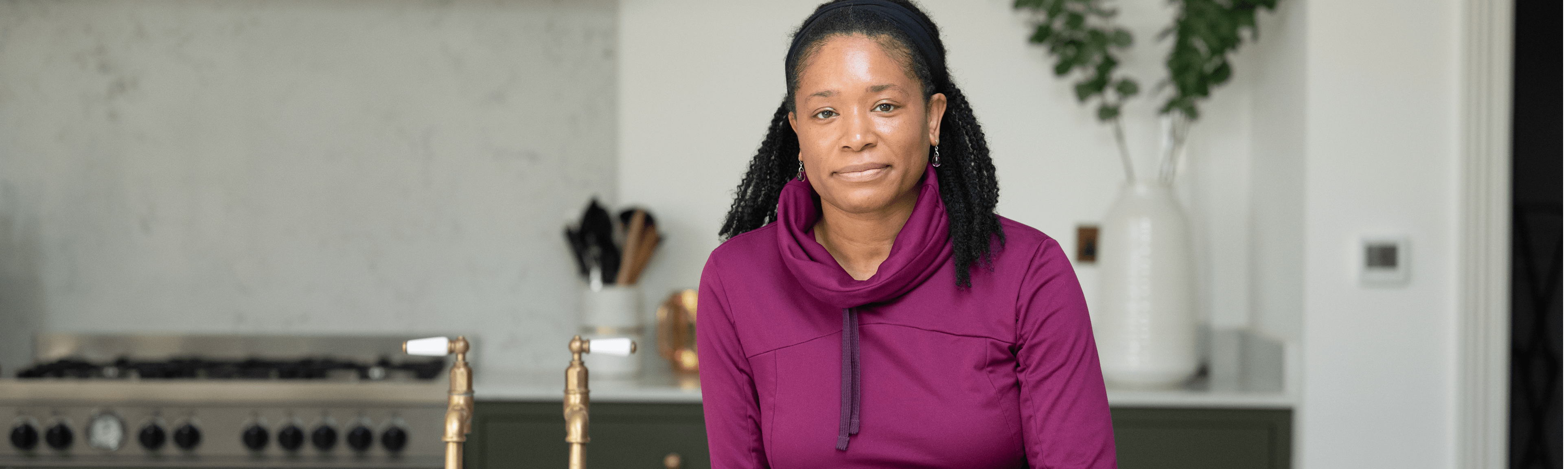
You will be redirected to an external site that may include social media, event registration, or other third-party content related to Alnylam.
Please note that these external sites have their own terms of service and privacy policies, which may differ from those of Alnylam.
Proceed to Site