Our Pipeline
Our robust pipeline of investigational RNAi therapeutics is currently focused on several disease areas including transthyretin amyloidosis, rare diseases, cardiovascular diseases, metabolic diseases, and neurological diseases.
Alnylam Grant Information
Alnylam is committed to supporting the medical and scientific understanding of our therapeutic areas of interest for healthcare professionals. Additionally, we are committed to supporting educational programs and initiatives spearheaded by organizations focused on patient advocacy, community education and support, and other scientific efforts related to our therapeutic areas of interest. To learn more about grants, sponsorships, or how to submit a grant request, visit our grants page.
Access to Investigational Medicines
Alnylam is dedicated to developing new therapies that have a positive impact on patient health, and to serving patients, patient families, and patient communities through education, empathy, and awareness. We understand that there are seriously ill patients who will not be eligible for our clinical trials and may not have options for alternative therapies, including investigational therapies in trials being conducted by other sponsors. In these circumstances, Alnylam will consider providing a requesting physician with pre-approval access to a specific Alnylam investigational medicine, for the treatment of an individual patient outside of a clinical trial, when certain conditions are met.
To read our statement on access to investigational medicines, click here.
Clinical Trials
Learn more about Alnylam-sponsored clinical trials your patients may be eligible for.
Alnylam Act®
Through Alnylam Act®, healthcare professionals can request genetic testing and counseling for hATTR amyloidosis, acute hepatic porphyria, or primary hyperoxaluria type 1 for individuals meeting certain criteria.
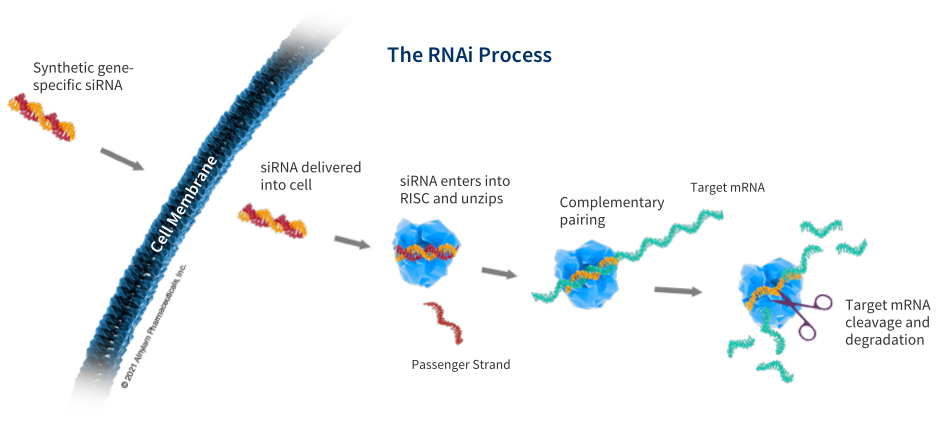
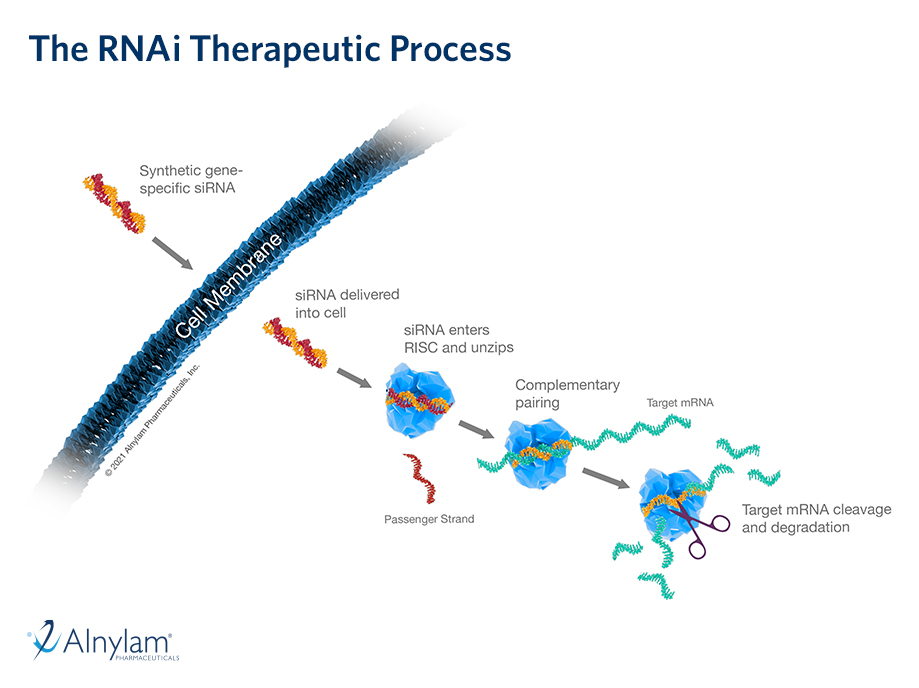
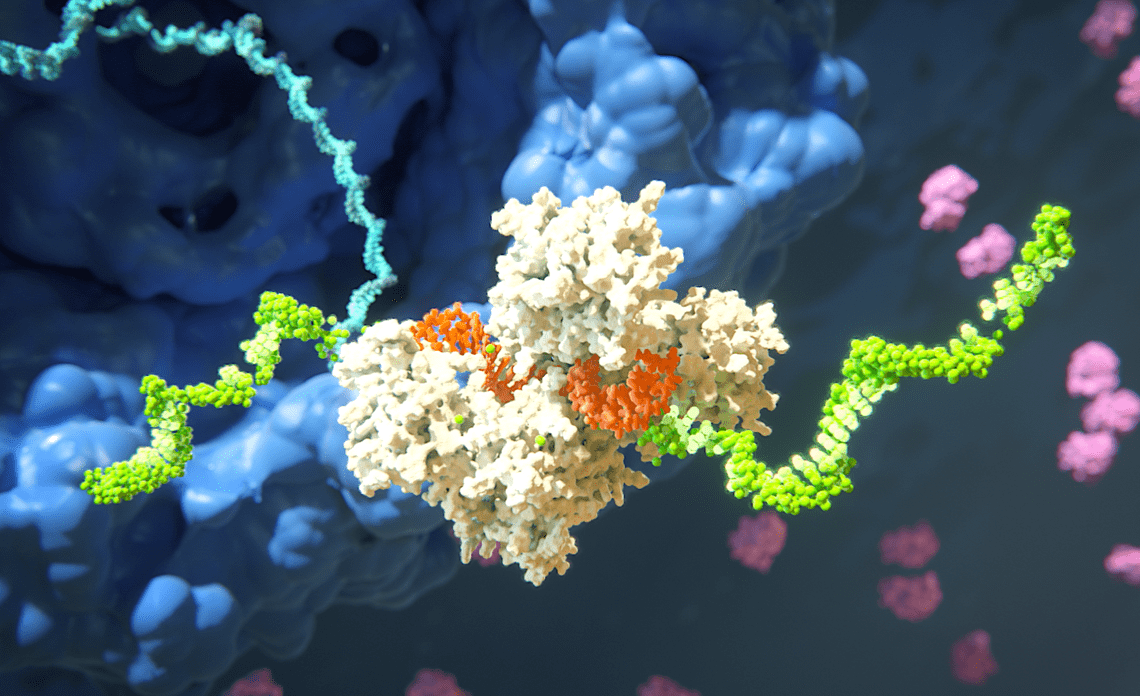
The Science Of RNAi
RNAi has helped create a new approach to developing medicine for genetic diseases.




You will be redirected to an external site that may include social media, event registration, or other third-party content related to Alnylam.
Please note that these external sites have their own terms of service and privacy policies, which may differ from those of Alnylam.
Proceed to Site